
Pioneering the development of second-generation non-replicative adenoviral vectors, employing a non-replicative and safely engineered Adenovirus 5-based vector (ΔE1 and ΔE3). Ensuring heightened safety standards and minimizing potential adverse effects.
Achieving High-immunogen expression on specific tissues and cell types through advanced Capsid Protein Engineering, including Fiber, Hexon, and Penton.
Rigorous selection of promoters, terminator, ex. for immunogen high expression in the target tissues.
Conducting a comprehensive professional literature analysis to inform and guide the rational design of immunogens.
Employing cutting-edge AI tools such as AlphaFold, RoseTTAFold, etc., to facilitate an AI-assisted immunogen design process.
Employing Molecular Dynamics simulations and QM methods to assess the structural stability and behavior of designed immunogens.


Combining insights from AI-driven methodologies with ongoing refinement based on Molecular Dynamics and Quantum Mechanics assessments.
Rigorous testing and validation of designed immunogens to ensure safety, efficacy, and compatibility with targeted tissues and cell types.
Streamlining immunogen design through the integration of AI, literature analysis, and advanced computational evaluations for expedited development of cutting-edge vaccine candidates.
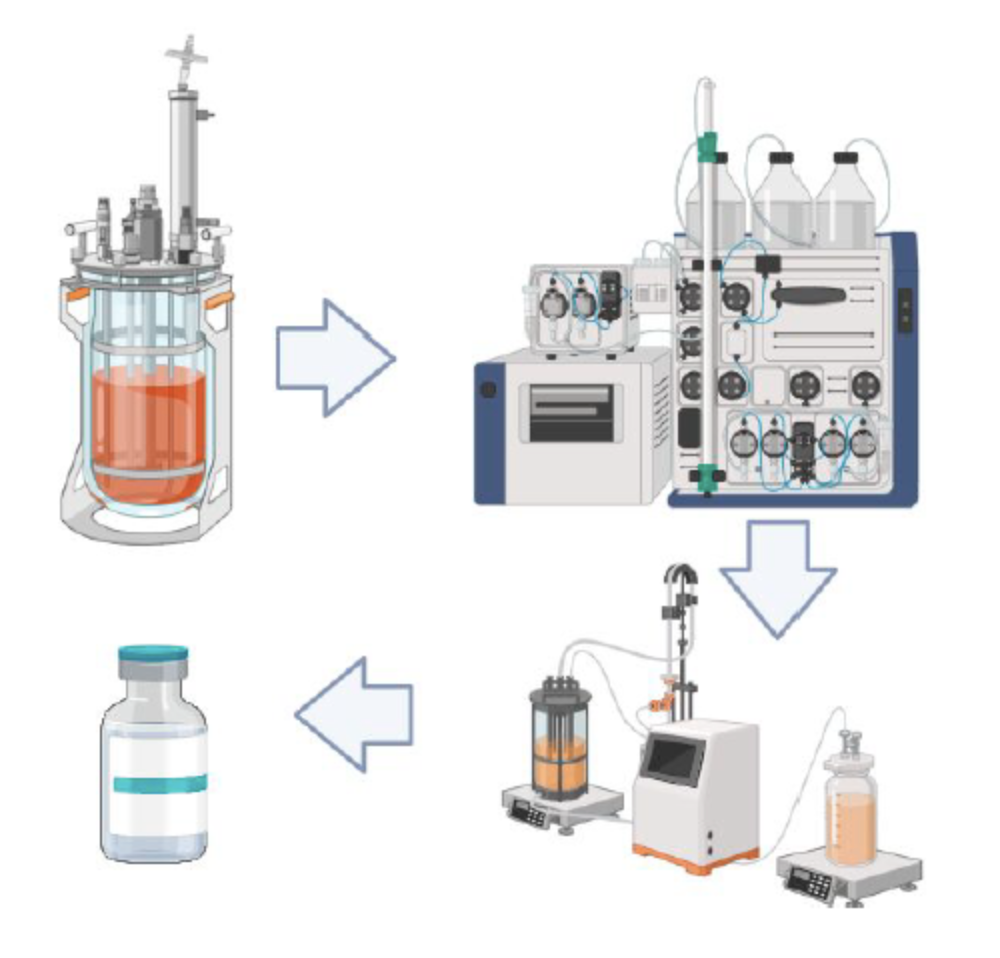
Establishing a small-scale setup for downstream and upstream development processes.
By being able to produce vaccine batches produced under scalable and transferable processes
Thanks to being able to develop and transfer production processes suitable for Industrial Production
Assembling a dedicated product development team to drive innovation, regulatory compliance, and seamless technology transfer from early development to large-scale manufacturing.
Application #63230284
48 Santo Amaro ST, APT 22, Sao Paulo, 04501-00, Brazil
Copyright 2024 © All rights Reserved.